Folks, I've just completed one of the most important scientific experiments of the century in an attempt to partially answer a question that has troubled humanity since antiquity: How do you keep a glass of chardonnay cool on a hot day?
 In late December 2025 I purchased a set of eight 3cm diameter spherical metal ice cubes
(pictured on the right). I was somewhat disappointed to discover that the spheres are too light
to be made of solid metal, and if you shake them you can hear a sloshing sound, so they must
contain some liquid and gas. Someone on Youtube can be seen drilling one open and he verifies
that it contains some kind of liquid gel and some air.
In late December 2025 I purchased a set of eight 3cm diameter spherical metal ice cubes
(pictured on the right). I was somewhat disappointed to discover that the spheres are too light
to be made of solid metal, and if you shake them you can hear a sloshing sound, so they must
contain some liquid and gas. Someone on Youtube can be seen drilling one open and he verifies
that it contains some kind of liquid gel and some air.
It seemed perfectly sensible to me that freezer temperature metal balls placed in a glass of cold white wine must help maintain a lower temperature for longer, even if they're not solid metal. I suspected that traditional ice cubes would be more effective, but diluting a nice chardonnay with melting ice is a venial sin that will get you ejected from most respectable social events.
You will find many YouTube videos of nerds comparing the relative temperature trends of liquids cooled by various means (including metal ice cubes), but I felt compelled to run a similar experiment with specific boundary conditions for typical cold white wine consumption.
The experiment required three identical glasses of chardonnay taken from the fridge, all starting at 7.1°C;
- A control glass containing only liquid.
- A glass with one 3cm metal sphere added (taken from the freezer).
- A glass with two small regular ice cubes added.
The resulting temperature changes over time were used to produce a scatter-plot in Microsoft™ Excel. Here are the results:
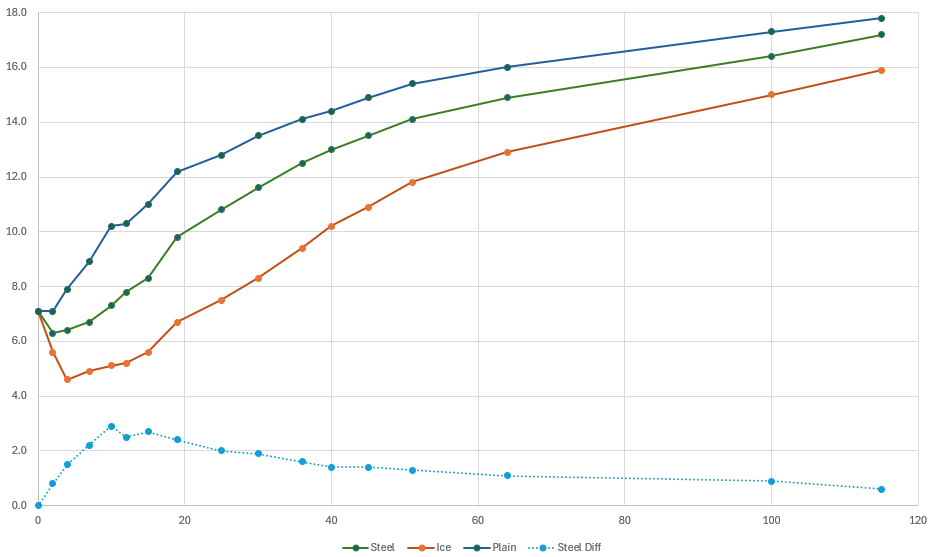
Observations
- Ice is technically superior – (orange series) I suspected this would be the case, and it's confirmed by this Wikipedia page on Specific Heat Capacities that water is the most commonly available and stable substance that has the highest heat capacity. As previously stated, you can't take advantage of this wonderous property of water and ice though, because it dilutes your chardonnay.
- Metal is second best – (green series) Also as I suspected, the cold metal spheres consistently keep the liquid colder than the control sample, but the modest improvement of 2-3°C only lasts for about 20 minutes, after which the difference shrinks and would be barely noticeable (light blue series). Since it takes around 10-15 minutes to consume a typical glass of chardonnay, the benefit of the cold metal is luckily felt during the initial critical time period.
Unknowns
- The experiment was done at room temperature, so it's unknown how much the shape of the three series would change if it all happened outdoors on a hot summer day.
- Would a solid metal sphere perform better than the hollow one? A solid 4cm diameter ball bearing costs about $18AU, so I'll add one to my next online order and update the results with a new data series.
- What about Dry Ice? A chunk of frozen CO2 at -40°C should act as a fabulously effective cooling agent which will not alter the taste or concentration of the chardonnay. The furious bubbles produced by the sublimation of the dry ice might be irritating, or entertaining, depending upon your attitude. I hope to add a data series for this as well sometime soon (I think there are companies around Cheltenham that sell blocks of dry ice).
