Contents: Greg's Element Collection ♦ Collection Density ♦ Densities ♦ Melting Points ♦ Electron Shells
|
H Hydrogen is a colourless gas and would be an uninteresting collection item.
He Helium is a colourless inert gas.
|
|||
.jpg)
|
LiLithium
10g in argon gas in a 30×120mm round bottom ampule. Since Lithium's density is so low (about half that of water), 10g of the element is
a considerable volume. As the picture shows, the ampule is quite large and it contains about 80 pieces of Lithium metal that seem have
been chopped from a ribbon. Notice how the shiny metal surfaces have been preserved by storage in Argon gas. If the pieces were
exposed to air they would completely tarnish to an unattractive dark grey colour in a matter of hours. Your muscles are tricked when you
pick up the ampule, as it looks like it's full of heavy iron or steel, but it's as light as wood.
|
03 |
W → 6.938 D → 0.534 MP → 180.5 ℃ BP → 1330 ℃ [He] 2s1 |
.jpg)
|
BeBeryllium
Beryllium is the first element in the
periodic table that is solid and stable at room temperature (after quite reactive lithium).
Its low density, small atomic radius, high melting point and metallic properties make it useful in a variety of exotic
applications in the aeropace industry (see: James Webb Telescope).
It's almost transparent to X-rays and it makes many unusual and desirable alloys.
Beryllium is only slightly denser than magnesium, so although the pellets look like lumps of heavy metal, they're as light as wood.
Beryllium is a rare and expensive element. A 1" cube costs a whopping $900 and it's about $2770 per kilogram.
My 10g sample was a more tolerable but still relatively high $33.
|
04 |
W → 9.0121831 D → 1.85 MP → 1287 ℃ BP → 2469 ℃ [He] 2s2 |
.jpg)
|
BBoron
5g in argon gas in a 15×60mm flat bottom ampule. It's hard to say anything really nice about Boron.
Even its name sounds a bit ugly. Although its a vital component of many compounds used in the modern world, most people probably forget that Boron
is an element or what the word means. As the Wikipedia article explains,
isolating pure Boron is technically challenging. To say something nice, the particles of Boron in the ampule are like glossy fine black
sand and they roll around pleasantly.
Futurama is brought to you by... Molten Boron!
Nobody doesn't like Molten Boron! |
05 |
W → 10.806 D → 2.08 MP → 2076 ℃ BP → 3927 ℃ [He] 2s2 2p1 |
.jpg)
|
CCarbon
Carbon in the allotropic form of
graphite is one of the softest substances known,
but this cube is surprisingly hard and it even feels a bit metallic. Rubbing the faces quite hard with a tissue barely leaves any stain
on the paper, so it wouldn't make very good pencil "lead". The cube feels as light as wood, but at 30g it's actually slightly heavier
than the 28g magnesium metal cube.
|
06 |
W → 12.011 D → 2.267 MP → ★ BP → 364 ℃ [He] 2s2 2p2 |
|
N Nitrogen is a colourless gas.
O Oxygen is a colourless gas.
F Flourine is a pale yellow highly reactive poisonous gas. Samples are very difficult to obtain or store.
Ne Neon is a colourless inert gas.
|
|||
.jpg)
|
NaSodium
After weeks of web searches, emails and phone calls I finally managed to purchase some
sodium metal from a company that supplies
science educational facilities. Sodium is fractionally less dense than water, so 100g seems to make large chunks which
look like a cheese round cut in half. The sample is stored in paraffin oil because sodium metal has the famous tendency
to generate hot hydrogen gas and explode on contact with water.
It's most enjoyable to cut thin slices off the chunks to reveal the shiny metal surface, which tarnishes in front of your eyes
and within a few minutes has turned light grey
(see YouTube Video).
|
11 |
W → 22.99 D → 0.968 MP → 97.79 ℃ BP → 882.94 ℃ [Ne] 3s1 |
.jpg)
|
MgMagnesium
Magnesium is the 6th lightest element,
but it's the lightest one an average person is likely to encounter, as the other 5 (Lithium, Potassium, Sodium, Rubidium and Calcium)
are highly reactive, rare or really expensive. It's a delight to toss the cube around in your hand and believe it's a real metal that's
used in alloys in planes, racing cars and bicycles. Burning magnesium produces stunning heat and white light and the only practical
way to extinguish the fire is to bury it in sand (read about the
1955 Le Mans disaster). I polished the cube to be mirror smooth,
but in a few days it was completely tarnished again. Pure magnesium is quite reactive, it will slowly release bubbles of hydrogen gas on contact with warm water.
|
12 |
W → 24.304 D → 1.738 MP → 650 ℃ BP → 1091 ℃ [Ne] 3s2 |
.jpg)
|
AlAluminium
Aluminium is everywhere in everyday life:
in planes, cars, building frames, cooking utensils, cans and foil (not tin foil ).
Although in those cases it's usually alloyed with a few percent of other metals to increase the strength
(pure aluminium is too soft for structural use).
Pure aluminium is noticeably "white" compared to the dull appearance of most other metals
(this graph explains why).
The cube is 55% heavier than the magnesium one, but it's still amazingly light to toss around in your hand. Iron is almost 3 times heavier.
|
13 |
W → 26.981 D → 2.7 MP → 660.32 ℃ BP → 2470 ℃ [Ne] 3s2 3p1 |
|
|
SiSilicon
The Silicon chunks are surprisingly attractive.
They look like pieces of dark silver reflective metal, but if you toss a few pieces around in the palm of your hand they feel more
like plastic and make a dull "dink" noise as they bump each other. I'm so impressed by silicon that I would love to own a
polished 1" cube
for its sheer alien beauty, but the current price of around $140 with $25 postage would be an indulgence I couldn't justify.
Look for images and videos of large pure silicon objects in web searches and you will appreciate the strange beauty of this element.
Silicon is the 2nd most abundant element in the Earth's crust after oxygen.
|
14 |
W → 28.084 D → 2.329 MP → 1414 ℃ BP → 3265 ℃ [Ne] 3s2 3p2 |
.jpg)
|
PPhosphorus
1g in argon gas in a 15×60mm flat bottom ampule. This is the more stable violet allotrope of Phosphorus. The small fragments look a bit
like roughly broken glass, but if you jiggle them around in the ampule they sound more like bits of plastic. Phosphorus comes in many
allotropic forms and they are all dangerous, poisonous or flammable to various degrees, so the pure element is regulated by most governments.
Phosphorus is a vital trace element in animal and plant life. Most Phosphorus is used in fertiliser production.
The parcel was tagged as Opened for inspection by Border Force (I hope they learned something).
|
15 |
W → 30.973 D → 2.36 MP → ~590 ℃ (?) BP → 620 ℃ [He] 2s2 2p1 |
.jpg)
|
SSulfur
I was surprised to see sulfur
sold as cubes. The Luciteria
site says they pour molten sulfur into molds where it vaguely holds the required shape,
but becomes crumbly when it cools. You can see burred edges in my sample cube. Sulfur is plentiful in the Earth's crust, so the 400g
bag of powder from Billing Metals only cost $12.
Pure sulfur has a very faint scent, but after handling the cube I noticed my fingers had a peculiar stronger smell
that took me back to my childhood chemistry set days (when sulfur was called sulphur). I presume oily fingers react very slightly
with the sulfur to make traces of some smelly compound.
Look at the surface of Jupiter's moon Io.
|
16 |
W → 32.059 D → 2.07 MP → 115.21 ℃ BP → 444.6 ℃ [Ne] 3s2 3p4 |
|
Cl Chlorine is a pale green highly reactive poisonous gas. Small sample ampules cost about $45.
Ar Argon is a colourless inert gas.
|
|||
.jpg)
|
KPotassium
10g of Potassium in an argon gas filled ampule to
preserve its natural metallic appearance. Exposed to air it would quickly tarnish and then slowly crumble to powder.
Potassium's density of only 0.86 g/cm³ makes it the 2nd lightest solid element after lithium at 0.53. So potassium would
float on water, but you can't demonstrate that of course because a violent explosion would result
(see This Video on the Periodic Table of Videos).
|
19 |
W → 39.098 D → 0.859 MP → 63.5 ℃ BP → 757.643 ℃ [Ar] 4s1 |
.jpg)
|
CaCalcium
Most people would associate Calcium with powdery and white substances like chalk, bone and milk.
But in fact, as the picture show, elemental Calcium is a shiny metal. The reason the 1cm cube sample looks so metallic is because it's sealed in a glass ampule
in Argon gas to preserve its appearance. If the cube were exposed to air it would rapidly tarnish and then slowly oxidise and crumble to powder.
|
20 |
W → 40.078 D → 1.55 MP → 842 ℃ BP → 1548 ℃ [Ar] 4s2 |
.jpg)
|
ScScandium
This 1cm cube of Scandium cost about $130 and is the most expensive sample added
to the collection by late 2024. The current trading price ranges from about $5000 to $20000 per kg depending on the purity.
Scandium is not that rare in the Earth's crust, but it isn't found in concentrated deposits, so extracting it is expensive.
If scandium was plentiful then it would be a gift to mankind due to its strength, low density and durability. But sadly, it's not the
case and scandium is used in small quantities alloyed with aluminium in high performance applications like aerospace and sports equipment.
|
21 |
W → 44.956 D → 2.989 MP → 1541 ℃ BP → 2836 ℃ [Ar] 3d1 4s2 |
.jpg)
|
TiTitanium
Lighter than iron or steel, stronger and more resitant to corrosion and heat ... no wonder two thirds of all
titanium produced is used in the aerospace industry
(an A380 Airbus contains 77 tons of titanium). The most titanium you'll ever see is in the cladding of the
Guggenheim Museum in Bilbao, Spain.
The polished titanium cube is a lovely thing to hold, and it tricks you when you pick it up, you think it's going to be as heavy as
a similar looking piece of iron or steel, but it's about half the weight. Titanium is the 9th most abundant element in the Earth's crust
at 0.5%, which means it's plentiful in many minerals, but extracting and refining it is a complex and expensive process.
|
22 |
W → 47.867 D → 4.506 MP → 1668 ℃ BP → 3287 ℃ [Ar] 3d2 4s2 |
.jpg)
|
VVanadium
Vanadium makes light and strong alloys.
Its first industrial use was in the Ford Model T car chassis.
Our expensive top quality victorinox kitchen knife is vanadium steel.
I would love to add a 1" cube of vanadium to the collection to join its fellow transition metal cubes, but the price of around $170 is
intolerable and I settled on getting 10g of metal fragments for a more reasonable $12. My fragments arrived tarnished with dark grey and rainbow colours,
which really surprised me because vanadium is supposed to be a corrosion resistant shiny silver metal. I soaked the pieces in vinegar for 10 minutes
and scrubbed them with a toothbrush and their appearance improved dramatically, as seen in the photo.
|
23 |
W → 50.94 D → 6.11 MP → 1910 ℃ BP → 3407 ℃ [Ar] 3d3 4s2 |
.jpg)
|
CrChromium
Anyone who owns a nice set of cutleries has probably touched pure
chromium metal.
Unalloyed chromium is so hard and corrosion resistant that it's deposited onto other metals as a thin layer via
electroplating.
The cube feels about the same weight as the iron one and the unpolished metal has a vague bluish tinge to it.
I polished the back of the nickel cube with a 1200 diamond grit plate and it almost becomes mirror smooth.
|
24 |
W → 51.9961 D → 7.19 MP → 1907 ℃ BP → 2671 ℃ [Ar] 3d5 4s1 |
.jpg)
|
MnManganese
The thin brown Manganese fragments are smooth on one
side and like rough sandpaper on the other. I think they are
electrolytically refined chips.
They don't look like metal, but if you toss some pieces around in your hand you can hear the metal-on-metal clinking sound.
Manganese is expensive because it's technically difficult to purify and handle at high temperatures.
For comparison, a 1" cube of chromium (element 24) costs only $33, but a 1" cube of manganese (element 25) costs $476 even though
it's 10 times more plentiful in the Earth's crust than chromium.
|
25 |
W → 54.938 D → 7.21 MP → 1246 ℃ BP → 2061 ℃ [Ar] 3d5 4s2 |
.jpg)
|
FeIron
Modern human civilisation is practically built on iron.
Alloys of iron with carefully selected traces of other elements form every piece of important machinery in the world.
People are familiar with iron and steel, so the weight of the cube is close to what the eye and hand expect.
The unpolished iron has a very dull grey appearance. The first iron ever found and used by mankind was delivered in meteorites,
long before the Iron Age.
I like to remind people that the cube has extra novelty value because it's almost pure iron, something the average person is
unlikely to encounter because pure iron is useless as a building material and it's vulnerable to rust.
|
26 |
W → 55.845 D → 7.874 MP → 1538 ℃ BP → 2862 ℃ [Ar] 3d6 4s2 |
.jpg)
|
CoCobalt
The cobalt cube looks and feels similar to the iron cube.
Cobalt makes magnetic high-strength alloys and its salts are historically famous for giving a deep blue colour to glass and ceramics.
The joy of finally adding cobalt to the collection (after a 90 day postal delay) is that it completes the famous "magnetic three" ...
iron, cobalt and nickel, the classical ferromagnetic metals.
A strong magnet can pick up all three cubes in a vertical stack –
see This Picture
|
27 |
W → 58.933194 D → 8.90 MP → 1495 ℃ BP → 2927 ℃ [Ar] 3d7 4s2 |
.jpg)
|
NiNickel
Like chromium, nickel is hard, durable and corrosion resistant,
so it's used in electroplating and stainless-steel alloys. Nickel is a common ingredient of coinage, sometimes in very pure form
(the Canadian "nickel" coin was pure nickel up until about 1981). The cube feels similar to the iron one, but the unpolished surface
is shinier and more silvery than both the iron and chromium. Like the chromium cube, I polished the back face and it can be made
almost mirror smooth.
|
28 |
W → 58.693 D → 8.908 MP → 1455 ℃ BP → 2730 ℃ [Ar] 3d8 4s2 |
.jpg)
|
CuCopper
Copper is one of the metal elements
that the average person might encounter daily. Pure copper is used in plumbing, cooking pots, electrical wiring and circuit boards.
Copper is the second-best electrical conductor metal, second only silver, which is why copper wire is carrying most of the
electricity around the modern world (silver would be more efficient, but think of the cost!). The cube feels like the iron one,
but the distinctive colour of the pure copper is quite hypnotic, especially when it's freshly cleaned in vinegar and gently polished.
|
29 |
W → 63.546 D → 8.96 MP → 1084 ℃ BP → 2562 ℃ [Ar] 3d10 4s1 |
.jpg)
|
ZnZinc
Zinc is another metal that people
might unknowningly see in pure form, probably as the "galvanised" coating on chain link fences, guard rails and "tin" roofs.
I always imagined zinc as being a dismal metal, but the cube feels almost as heavy as the iron one, and it has a light silvery unpolished surface.
Zinc is an important trace element in the human body and its deficiency is associated with many diseases.
Brass is an alloy of about 60% copper and 40% zinc
(easily confused with bronze which is copper and tin).
|
30 |
W → 65.38 D → 7.14 MP → 419.53 ℃ BP → 907 ℃ [Ar] 3d10 4s2 |

|
GaGallium
The gallium arrived as 2 × 10g 'slugs' in two thin
plastic tubes. I warmed the tubes in hot water and poured the melted gallium into ice cube trays to mold little discs of various sizes.
Gallium is the vertical middle brother of aluminium
and indium, and its properties are vaguely an average of its brothers.
It's described as a metal, but it fails the metal pub test because it's soft enough to scratch with your fingernail
and—most disappointingly—it melts in your hand at the astonishingly low temperature of 29.7°C.
|
31 |
W → 69.723 D → 5.91 MP → 29.7646 ℃ BP → 2400 ℃ [Ar] 3d10 4s2 4p1 |
.jpg)
|
SeGermanium
The germanium fragments are attractive and shiny and look
a lot like their vertical family member Silicon.
Mendeleev predicted germanium existed before it was found,
he called the missing element ekasilicon. Germanium is a semiconductor
and is a technology critical element in the modern world. Germanium is quite expensive because it is rare in the Earth's crust and can only
be extracted from a small number of minerals.
|
32 |
W → 72.630 D → 5.323 MP → 938.25 ℃ BP → 2833 ℃ [Ar] 3d10 4s2 4p2 |
.jpg)
|
AsArsenic
Pictures of arsenic in inert gas show it be a
dull grey metalloid, but my fragments are as dark as charcoal so they're well oxidised. In the right light through a magnifying glass
I can see a few shiny spots that hint of the metallic surface underneath. Arsenic and its compounds are famous for being
poisonous and carcinogenic, and the small skull and crossbones on the shipping bottle was evidence for that.
|
33 |
W → 74.922 D → 5.727 MP → ★ BP → 615 ℃ [Ar] 3d10 4s2 4p3 |
.jpg)
|
SeSelenium
My selenium fragments are the black allotrope
that looks metallic but feels more like hard plastic. Wikipedia says that most selenium is used in glassmaking and pigments, and more surprisingly,
it's a vital trace element in all animal life and some plants. It's relatively cheap at about $200/kg.
|
34 |
W → 78.97 D → 4.82 MP → 221 ℃ BP → 685 ℃ [Ar] 3d10 4s2 4p4 |
|
Br Bromine is a dark red highly reactive poisonous liquid. A 1ml ampule costs about $50.
Kr Krypton is a colourless inert gas.
Rb Rubidium is a highly reactive soft metal. One gram in an argon gas ampule costs about $100.
|
|||
.jpg)
|
SrStrontium
The strontium fragments arrived completely
covered in grey oxide with pale yellow spots. I polished some of the flatter chunk surfaces to reveal the shiny metal surface underneath,
but strontium is so reactive that the clean surfaces tarnished and turned pale yellow in less than a minute. I tried polishing, wiping and
dropping into paraffin oil as quickly as possible to preserve the shine, but sadly, the fragments are completely tarnished again after about
30 minutes in the oil. I will have to find some alternative way of preserving clean strontium metal.
After two years in paraffin the chunks became completely coated in an ugly skin of pale white powder with the consistency of icing sugar
that had to be scraped off with a knife followed by sandpapering to reveal the metal. Web searches don't quickly reveal what the skin is,
maybe the oxide … stay tuned for news.
|
38 |
Sr → 87.62 D → 2.64 MP → 777 ℃ BP → 1377 ℃ [Kr] 5s2 |
.jpg)
|
YYttrium
The small (1cm) yttrium cube has a shiny silvery appearance
similar to the nickel cube. Like many other metals, it forms a thin oxide passivation
layer which protects it in normal conditions.
Yttrium is not normally listed in tables as part of the lanthanide
series, but it's always mixed with them in ores and it's chemical properties are very similar.
Yttrium cubes cost about the same as tungsten, but I felt no need for a $92 large cube of yttrium and was satisfied by purchasing
a smaller 1cm cube for a reasonable $18.
|
39 |
Y → 88.90584 D → 4.472 MP → 1526 ℃ BP → 2930 ℃ [Kr] 4d1 5s2 |
.jpg)
|
ZrZirconium
I'm really embarrassed to admit that I forgot zirconium
was an element until I bought the cube, mixing it up with zirconia (probably thanks to an episode of
The Simpsons).
The cube feels and looks a bit like the nickel cube, but perhaps slightly more silvery. Zirconium is about as abundant as chromium,
nickel and zinc, but it seems to be little known to the general public. Zirconium is normally tainted with a few percent of
hafnium, and the delicate process of separating
them was not considered important until the nuclear industry demanded they be separated so they could exploit their completely
different Neutron Cross-Sections.
|
40 |
W → 91.224 D → 6.52 MP → 1855 ℃ BP → 4377 ℃ [Kr] 4d2 5s2 |
.jpg)
|
NbNiobium
The 1cm cube of niobium looks a bit like the
yttrium and nickel cubes. Niobium is so chemically similar to tantalum that it took 50 years before chemists were sure they were separate elements.
Niobium makes heat resistant super-alloys. This Wikipedia page contains
a picture of a rocket booster nozzle made from an alloy of 90% niobium. Although it's little know to the general public, niobium has many
modern technological applications.
|
41 |
Ni → 92.90637 D → 8.57 MP → 2477 ℃ BP → 4744 ℃ [Kr] 4d4 5s1 |
.jpg)
|
MoMolybdenum
Molybdenum makes really hard and
heat resistant alloys, so it's used in things like drill bits, saw blades and rifle barrels.
The molybdenum cube looks very similar to the iron cube, slightly lighter grey and noticeably about 30% heavier.
Molybdenum's melting point of 2623℃ is the 4th highest, so I suspect that this cube is
sintered like the tungsten cube,
that is, it's made from metal powder compressed at very high temperature and pressure. The fact that it's 1.5% lighter than that theoretical
weight (just like the Tungsten cube) is supporting evidence that it's a sintered cube.
|
42 |
W → 95.95 D → 10.28 MP → 2623 ℃ BP → 4639 ℃ [Kr] 4d5 5s1 |
|
Tc Technetium has no stable isotopes. Samples are very rare and expensive.
Ru Ruthenium metal costs about $50 per gram.
Rh Rhodium metal costs about $420 per gram. It is the most expensive of the stable elements.
Pd Palladium metal costs about $220 per gram.
|
|||
.jpg)
|
AgSilver
No wonder silver has been considered one of the precious
metals of antiquity,
because although my pellets are small, they are very attractive and the smooth surfaces actually look "silver".
Only a handful of the elements are coloured, and silver is considered one of them. Silver is the best conductor of heat and electicity
and it has a wide variety of critical applications in the modern world.
|
47 |
W → 107.8682 D → 10.49 MP → 961.78 ℃ BP → 2162 ℃ [Kr] 4d10 5s1 |
.jpg)
|
CdCadmium
For some reason, cadmium often seems to be provided
as balls in sizes that fit in the palm of your hand. A web search showed that
Northern Smelters
were selling Cadmium balls for about $25 and I couldn't resist the urge to get one. This ball weighs 568g.
Cadmium is slightly denser than iron, so the ball is quite hefty to hold. Most of the world's cadmium is used in batteries, and some in electroplating.
This ball arrived quite "dirty" and rough, so polishing with increasingly finer grades of sandpaper have made it smooth and reflective.
Cadmium compounds are very poisonous so the polishing was done with thick gloves, outdoors,
at arms length, with a breeze blowing away from my body
.
|
48 |
W → 112.414 D → 8.65 MP → 321.07 ℃ BP → 767 ℃ [Kr] 4d10 5s2 |
.jpg)
|
InIndium
I purchased this 10g indium mini-ingot from
Billing Metals. Indium looks and feels like metal (it's only a fraction less dense than
iron), but it's a trick: it's so soft you can dent it with your fingernail, thereby failing the pub test for a real metal.
It's moderately expensive at about $500/kg, as it's abundance in the Earth's crust is close to those of silver and mercury. Several hundred tons of
indium are produced annually and half of it goes into LCD screens.
|
49 |
W → 114.818 D → 7.31 MP → 156.6 ℃ BP → 2072 ℃ [Kr] 4d10 5s2 5p1 |
.jpg)
|
SnTin
I have Tin samples as the 1" cube and pellets cut from wire (pictured) and a 496g bar
purchased from Northern Smelters, which was moderately expensive because
pure tin is not in common use and it's down at about 40th on the abundancy scale in the Earth's crust, just above uranium.
The bar makes a faint "creaking" sound when it's bent, but it's not the famous
tiny cry I was hoping for.
The purity of the bar is unknown, but the pellets from Billing Metals are 99.99% pure
and the cube is 99.9% pure. Note that "tin cans" were once made of a pure tin electroplated layer over steel, but modern alloys are making that
sort of can increasingly rare. If you own any pewter objects then they are probably about 90% tin.
|
50 |
W → 118.710 D → 7.265 MP → 231.93 ℃ BP → 2602 ℃ [Kr] 4d10 5s2 5p2 |
.jpg)
|
SbAntimony
The Antimony chunks look like an attractive shiny silver metal,
but if you toss some pieces around in your hand they feel and sound more like hard plastic. This is a good example
of an element that is a metalloid. Antimony is about as rare as mercury and silver
in the Earth's crust, but it's relatively cheap, perhaps because it's present in many minerals and is easy to extract.
|
51 |
W → 121.760 D → 6.697 MP → 630.63 ℃ BP → 1635 ℃ [Kr] 4d10 5s2 5p3 |
.jpg)
|
TeTellurium
My tellurium samples are clearly the allotrope
that is crystalline and looks like a silvery metal, but don't be fooled, it's a dismal metalloid that is so brittle that it crumbles
in your fingers. Tellurium is common in the universe but extremely rare in the Earth's crust, being about as rare as
platinum. It is however much cheaper than platinum: 1kg of Te is about $400 and 1kg of Pt is about $45500.
Most of the tellurium produced goes into iron and copper alloys to make them more machinable.
|
52 |
W → 127.60 D → 6.24 MP → 449.51 ℃ BP → 988 ℃ [Kr] 4d10 5s2 5p4 |
.jpg)
|
IIodine
Iodine is the least poisonous of
its "halogen" element family which includes deadly fluorine,
chlorine and
bromine.
A classic demonstration with Iodine is to heat some of the dark crystals and watch them sublimate into a dark purple gas,
then recrystalise the gas on a cold glass surface (see this video).
I forgot how volatile iodine is. After I took the usual element picture sample on 5mm ruled graph paper, I poured the grains back into the bottle
and noticed the paper was stained pale orange. I tore out the top sheet and noticed the one below was also stained, so I tore that out ... and so on for a
depth of 12 sheets of paper before the stain was acceptably dim. So keep iodine away from skin and precious objects.
|
53 |
W → 126.904 D → 4.933 MP → 113.7 ℃ BP → 184.3 ℃ [Kr] 4d10 5s2 5p5 |
|
Xe Xenon is a colourless inert gas.
Cs Caesium is a soft highly reactive metal. A 1g sample in an argon gas ampule costs about $90.
|
|||
.jpg)
|
BaBarium
Pure barium is a soft silver metal,
so clearly my chunks have been badly oxidised. I tried rubbing and scratching some faces to reveal the metal sufrace without
success before transferring them into paraffin oil. Barium is a bit of an outlier element that isn't in big demand, only being used
in some special alloys and the nitrate makes green fireworks. It's about as rare in the Earth's crust as sulfur and strontium,
but it's moderately priced at about $400/kg. Pure barium sulphate is ingested to contrast the digestive system in x-rays.
|
56 |
W → 137.328 D → 3.51 MP → 727 ℃ BP → 1845 ℃ [Xe] 6s2 |
.jpg)
|
LaLanthanum
The metal chunks in the picture have just been polished with sandpaper to remove the dull gray tarnish that will completely
cover them again within an hour or so. Immediately after taking the picture, the chunks were dropped back into their test tube of
paraffin oil in a futile attempt to preserve their natural shiny metal appearance. Sadly, the oil only delays the tarnishing for about
a week. Lanthanum gives its name to the lanthanide series
of elements which follow it, but there are disputes about it technically being one of the lanthanides because it does not have
any 4f shell electrons. Lanthanum is rather soft, as when I held the pieces in pliers for sandpapering, I could see little teeth
marks in the metal surface.
|
57 |
W → 138.905 D → 6.162 MP → 920 ℃ BP → 3464 ℃ [Xe] 5d1 6s2 |
.jpg)
|
CeCerium
Cerium is the most abundant
of the rare earth elements, but it still costs about $490/kg. My samples are terribly tarnished and have lost their
natural silvery appearance despite being stored at first in fine machine oil and now in paraffin.
Cerium is famous for being pyrophoric
and it's used in white LED light sources. I rubbed some edges of the pieces with sandpaper to reveal the shiny metal
surface, but they became dull grey again after a few days back in the paraffin. And I can confirm that cerium really is
pyrophoric ... bright sparks flew out of the sandpaper while I was sanding the pieces
(see the YouTube Video).
|
58 |
W → 140.116 D → 6.770 MP → 795 ℃ BP → 3443 ℃ [Xe] 4f1 5d1 6s2 |
.jpg)
|
PrPraseodymium
Professional pictures show praseodymium
to be a dark silvery metal, but since the samples arrived preserved in mineral oil it's a strong hint that they tarnish easily in air,
and my chunks look quite tarnished despite the oil. Wikipedia says the oxide tarnish is pale green, and I swear I can see a very faint
greenish tinge to the samples in this photo.
|
59 |
W → 140.907 D → 6.77 MP → 935 ℃ BP → 3130 ℃ [Xe] 4f3 6s2 |
.jpg)
|
NdNeodymium
The neodymium chunks arrived completely covered on black oxide despite being shipped in oil.
I polished some of the flatter surfaces of the chunks with sandpaper to reveal the silvery metal, then I quickly
put them into fresh paraffin oil before they tarnished again. In the photo you can see some of the metallic surface.
Neodymium is famous as a part of the alloy Nd2Fe14B which makes the
strongest permanent magnets known.
Ironically, neodymium itself ignores my strongest magnet.
|
60 |
W → 144.242 D → 7.01 MP → 1024 ℃ BP → 3074 ℃ [Xe] 4f4 6s2 |
|
Pm Promethium has no stable isotopes. No samples exist.
|
|||
.jpg)
|
SmSamarium
Internet pictures of samarium
show it to be a dark silvery metal, but they all look freshly cut or stored in inert gas. My small pieces are very dark grey so I
presume their surfaces are well oxidised. The only use for samarium I can find is that it makes powerful magnets that are heat
resistant due to the high Curie point.
|
62 |
W → 150.36 D → 7.52 MP → 1072 ℃ BP → 1900 ℃ [Xe] 4f6 6s2 |
.jpg)
|
EuEuropium
The Europium arrived in a small bottle of oil and all of the
chunks had a dark brown and yellow oxidised coating. Europium is one of the most reactive of the lanthanides, so I quickly sandpapered
the surfaces as best I could to expose the shiny metal and then wiped them and dropped them into the paraffin oil for preservation.
I was partially successful, as the picture shows some of the exposed shiny metal, and you can also see patches of pale yellow carbonate.
Europium is also quite soft, as gripping the chunks in pliers left little teeth marks on the surface.
|
63 |
W → 151.964 D → 5.264 MP → 826 ℃ BP → 1529 ℃ [Xe] 4f7 6s2 |
.jpg)
|
GdGadolinium
I learned in childhood that only 3 elements are attracted to a magnet: iron (found everywhere), nickel
(found in many coins) and cobalt (not in common use). It was only in 2020 I learned that
gadolinium is part of the magnetic
family ... and it's true! The gadolinium fragments in the tube leap to a magnet. Gadolinium is
ferromagnetic
below 20℃ and paramagnetic above.
That means that Gadolinium can be magnetised on a cold day, but demagnetises as the temperature approaches room temperature.
It's quite weird to see this unfamiliar substance powerfully attracted to a magnet.
|
64 |
W → 157.25 D → 7.90 MP → 1312 ℃ BP → 3000 ℃ [Xe] 4f7 5d1 6s2 |
.jpg)
|
TbTerbium
The Terbium metallic chunks look and feel like their neighbours.
Wikipedia says most terbium is used to make green phosphorescent lighting, but it also says it can be cut with a knife, and this
is clearly not true because the chunks feel quite hard. My strongest magnet can lift the chunks easily, which quite surprised me.
Terbium metal has a disordered paramagnetic state above 230K, so can someone explain what that means in practical terms?
|
65 |
W → 158.92535 D → 8.23 MP → 1356 ℃ BP → 3123 ℃ [Xe] 4f9 6s2 |
.jpg)
|
DyDysprosium
Wikipedia says that pure dysprosium
was not isolated until ion-exchange techniques were available in the 1950s, and it only has a few obscure practical applications.
The chunks are very weakly attracted to my strongest magnet.
|
66 |
W → 162.500 D → 8.540 MP → 1407 ℃ BP → 2562 ℃ [Xe] 4f10 6s2 |
.jpg)
|
HoHolmium
Wikipedia tells me that holmium
is famous for having the highest magnetic permeability
and highest magnetic moment of any element,
but can someone explain what means? The chunks are very weakly attracted to my strongest magnet.
|
67 |
W → 164.930 D → 8.79 MP → 1461 ℃ BP → 2600 ℃ [Xe] 4f11 6s2 |
.jpg)
|
ErErbium
Thanks to the Matter book, I've known that erbium
is an element since I was in primary school, but I never thought I'd see the element mentioned in popular culture until 1986 when Carl Sagan's book
Contact was published. In chapter 15 titled
Erbium Dowel,
part of the instructions to build
The Machine
require 96% pure erbium (a technical challenge), which seems quaint now that my sample is 99.9% pure.
The erbium dowels contribute to a major part of the plot, but you'll have to read the book to find out why.
The chunks are very weakly attracted to my strongest magnet.
|
68 |
W → 167.259 D → 9.066 MP → 1529 ℃ BP → 2868 ℃ [Xe] 4f12 6s2 |
.jpg)
|
TmThulium
Thulium is the least abundant stable lanthanide so the 10g fragments cost
a moderately high $25. Wikipedia says that thulium can be cut with a knife, but I can barely dent the surface with a blade point. It also says
thulium slowly tarnishes in air, but my pieces are quite shiny, so I guess they've been recently manufactured or cut from a larger sample.
The pieces are very slightly attracted to my most powerful magnet.
Thulium's only narrow applications seem to be in infra-red
lasers and isotope 170Tm as an x-ray source. The first pure sample was isolated in 1911 after a long and complex process.
|
69 |
W → 168.934218 D → 9.32 MP → 1545 ℃ BP → 1950 ℃ [Xe] 4f13 6s2 |
.jpg)
|
YtYtterbium
Pictures of ytterbium
on the internet show it to be bright and silvery when it's freshy cut or stored properly.
Ytterbium supposedly reacts slowly with air and forms a dark tarnish, but luckily my pieces still retain a metallic look.
It's one of the less abundant rare earths, and a pure sample wasn't isolated until 1953.
|
70 |
W → 173.045 D → 6.90 MP → 824 ℃ BP → 1196 ℃ [Xe] 4f14 6s2 |
.jpg)
|
LuLutetium
The arrival of 10g of Lutetium in June 2021 means I now have a complete
set of lanthanides
(except radioactive promethium). My 1969 Matter books says of lutetium:
"A pound of pure lutetium costs £450. With many of its chemical and physical properties unknown, it has no practical value".
Time has been a little bit kind to lutetium, because since then it's been found that lutetium isotopes can be used for deep-time dating
and radionuclide therapy. Otherwise, it's rare and doesn't have any
other attractive properties. The cost has gone up too, as one pound (454 gram) costs about $2500 at today's prices.
|
71 |
W → 174.9668 D → 9.841 MP → 1652 ℃ BP → 3402 ℃ [Xe] 4f14 5d1 6s2 |
.jpg)
|
HfHafnium
The hafnium pellets have a dark silvery attractive
appearance and feel noticably heavy for their size. As explained in the zirconium
entry above, hafnium and zirconium are always found together and the difficult task of separating them was not important
until the 1950s when the nuclear industry required pure samples of both elements.
Hafnium was the second last stable element to be discovered in 1923 (rhenium was last in 1925).
Hafnium is mainly used in control rods in nuclear power plants due it's large
neutron capture cross section.
|
72 |
W → 178.486 D → 13.31 MP → 2233 ℃ BP → 4603 ℃ [Xe] 4f14 5d2 6s2 |
.jpg)
|
TaTantalum
Tantalum is hard blue-grey metal with high density,
high melting point and high corrosion resistance. I would love to have a 1" tantalum cube to add to my collection, but its rarity
in the Earth's crust (similar to germanium and beryllium) pushes the price up to about $520. A kilogram costs about $1250.
I was satisfied to buy 10g of fragments for about $14. Tantalum is a vital component in modern computers, phones and other electronic
equipment. The pliability and inertness of tantalum foil makes it useful for surgical implants.
|
73 |
W → 180.948 D → 16.69 MP → 3017 ℃ BP → 5458 ℃ [Xe] 4f14 5d3 6s2 |
.jpg)
|
WTungsten
The party trick is to pick up the 28g magnesium
cube (density 1.74) and toss it around in your hand, then pick up the 310g tungsten
cube (density 19.3) and be shocked by the weight difference factor of about × 11. Everyone should know that a tungsten wire filament is used in
classic lightbulbs where it's heated to about 3000℃ and it emits a near continuous spectrum of visible light. Tungsten has the highest melting
point of all metals at 3422℃, so this cube is not cast from molten tungsten, it's
sintered from powdered tungsten at very high temperate
and pressure and is about 1.5% less dense than expected.
|
74 |
W → 183.84 D → 19.3 MP → 3422 ℃ BP → 5930 ℃ [Xe] 4f14 5d4 6s2 |
|
Re Rhenium metal costs about $90 for a 10g bead.
|
|||
.jpg)
|
OsOsmium
This tiny 1g sample of Osmium was melted into a 5mm diameter bead
by an electric arc, which the only way to melt it due to its very high melting point (3rd hightest of all metals).
What makes Osmium so famous though is its density, which is the highest of all elements at 22.59 g/cm3, twice the density of lead.
I can feel the density when I jiggle the bead around in the plastic test tube. Web searches hint that the annual worldwide production of
Osmium is about 500 to 1000 kg (1 tonne).
The bead is too small to reveal the pale bluish tint of the metal. The price of the 1g bead was about $60. The price of a 1cm cube is about
$1,600 and the price of a 1 inch cube is about $19,600 (in January 2025).
|
76 |
W → 190.23 D → 22.587 MP → 3033 ℃ BP → 5008 ℃ [Xe] 4f14 5d6 6s2 |
|
Ir Iridium metal costs about $320 for a 1g bead.
Pt Platinum metal costs about $85 per gram.
|
|||
.jpg)
|
AuGold
Yes, I have added gold in the collection. But I cheated!
This is $5 worth of crumpled gold leaf from the supermarket. Even this tiny quantity reveals why gold has been the most prized
precious metal since antinquiy: it's colour is hypnotic. I emphasised the word colour because it doesn't simply have a
colour, you're seeing "plasma oscillations in the valence electrons in the visible range due to relativistic
effects in the orbitals of gold atoms" [Wikipedia].
As I type this (Aug 2024), adding a 1" cube of gold to my collection would cost about $41,500. If you want to see a ridiculous
amount of gold, watch Gold Bullion Vault - Periodic Table of Videos.
|
79 |
Au → 196.966570 D → 19.3 MP → 1064.18 ℃ BP → 3243 ℃ [Xe] 4f14 5d10 6s1 |

|
HgMercury
Mercury is the most fantastic
and bizarre element that a person has a reasonable chance of encountering. It is literally "liquid metal" at room temperature.
It's mesmerising to pour mercury and watch heavy silver streams and beads flow like something from a science fiction movie.
I spent weeks trying to buy some mercury for the collection, but it's only available in commercial quantities like a 25kg minimum,
and it's far too expensive and dangerous to own that much mercury. I obtained 1 cubic inch (222g) of mercury thanks to a donation
from friend who collected a large amount decades ago.
|
80 |
W → 200.592 D → 13.534 MP → -38.829 ℃ BP → 356.73 ℃ [Xe] 4f14 5d10 6s2 |
|
Tl Thallium is a poor metal which easily forms highly poisonous salts. 10g in an argon ampule costs about $220.
|
|||
.jpg)
|
PbLead
Lead is the densest material that
the average person will handle, hence expressions like "heavy as lead" or "lead balloon". It's only 18th on the density scale,
but everything higher is rare or expensive. Lead is 44% heavier than iron. I also purchased a 692g bar of lead from
Northern Smelters, which can be polished with fine sandpaper to
rereveal the true shiny metal surface of clean lead, but within a week the surface becomes terribly tarnished.
The cube from Luciteria is 99.95% pure. I also attempted to polish the cube sides and back, but lead is so soft that the super-fine abrasive
left countless small streaks. I'll have to find another polishing technique.
|
82 |
W → 207.2 D → 11.34 MP → 327.46 ℃ BP → 1749 ℃ [Xe] 4f14 5d10 6s2 6p2 |
.jpg)
|
BiBismuth
Bismuth is on the borderline
of being what the average person might consider a "metal". Superficially it feels metallic, and it's almost as heavy
as lead, but as this 1.19kg sample chunk shows, when it's struck it tends to break and reveal the internal crystalline structure.
You can melt bismuth on your stove and it looks like molten lead. Look for videos of people making bismuth "crystals" as a hobby.
It's not quite as dense as lead, but the chunk in the picture is much heavier than your eye and hand expect when you pick it up
(it's 24% heaver than iron). Bismuth has the peculiar fame of being the highest atomic number "stable"
(aka primordial)
element after which they all go to radioactive hell … although, there is a nitpicking
technical dispute about that claim.
|
83 |
W → 208.980 D → 9.78 MP → 271.5 ℃ BP → 1564 ℃ [Xe] 4f14 5d10 6s2 6p3 |
|
All elements starting at atomic number 84 (polonium) and higher are radioactive. In the US it is legal to own up to 7kg of U238 (depleted uranium) or thorium, but none can be exported outside the US (see 10 NRC§40.22). It is unknown if U238 or thorium are available in Australia for private ownership. Web searches on the subject produce no useful information. |
|||

|

|
| The famous 3 ferromagnetic elements | All the metal cubes (and Sulfur) |
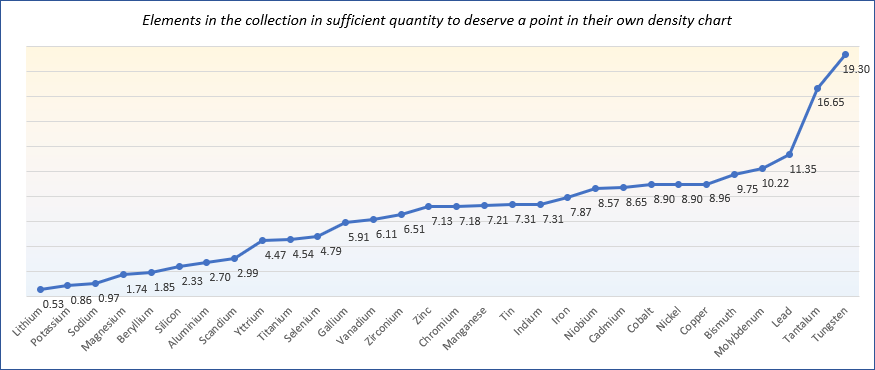
The following chart shows the decreasing densities of all the solid elements.
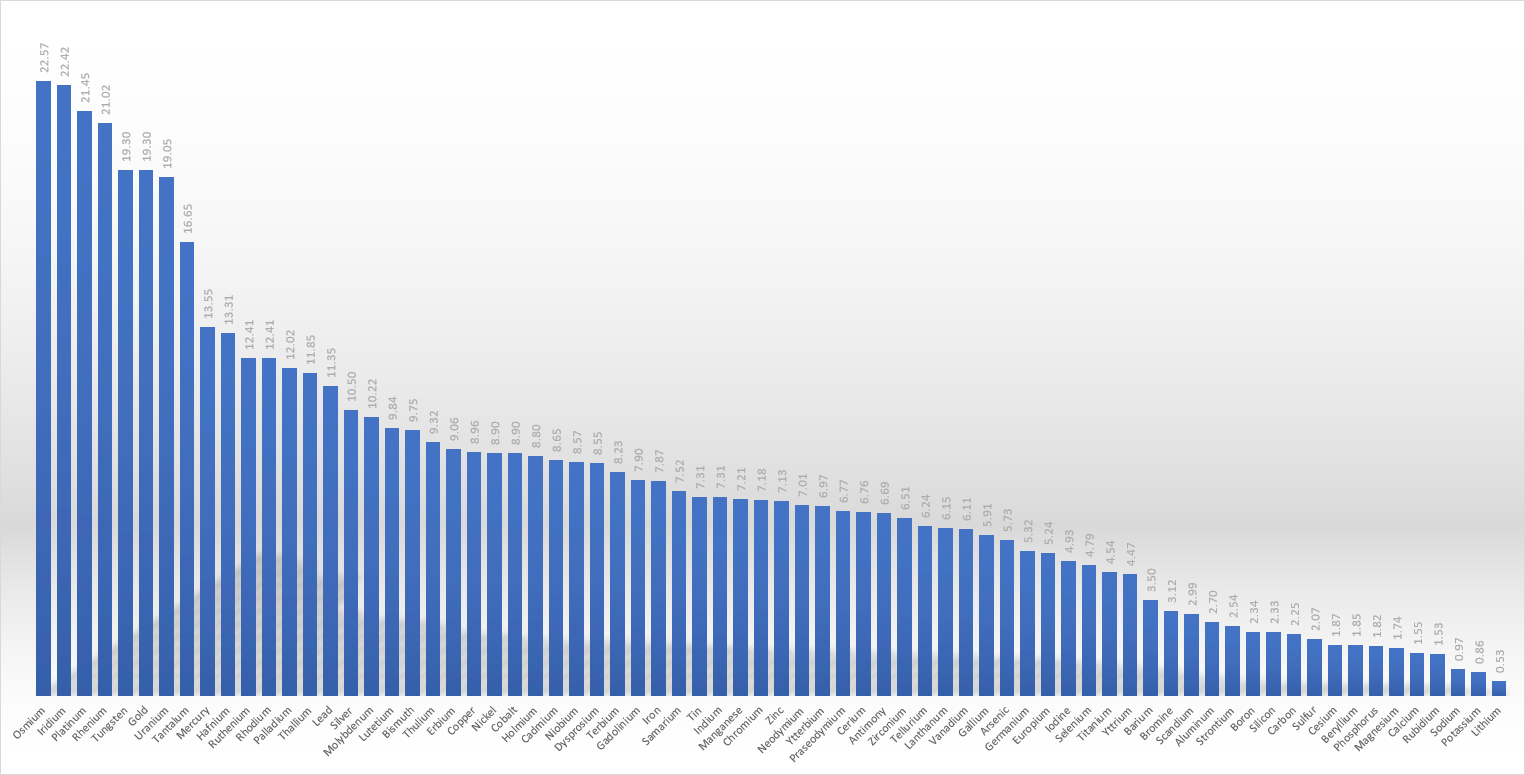
The following chart shows the decreasing melting points of all the (mostly) solid elements.
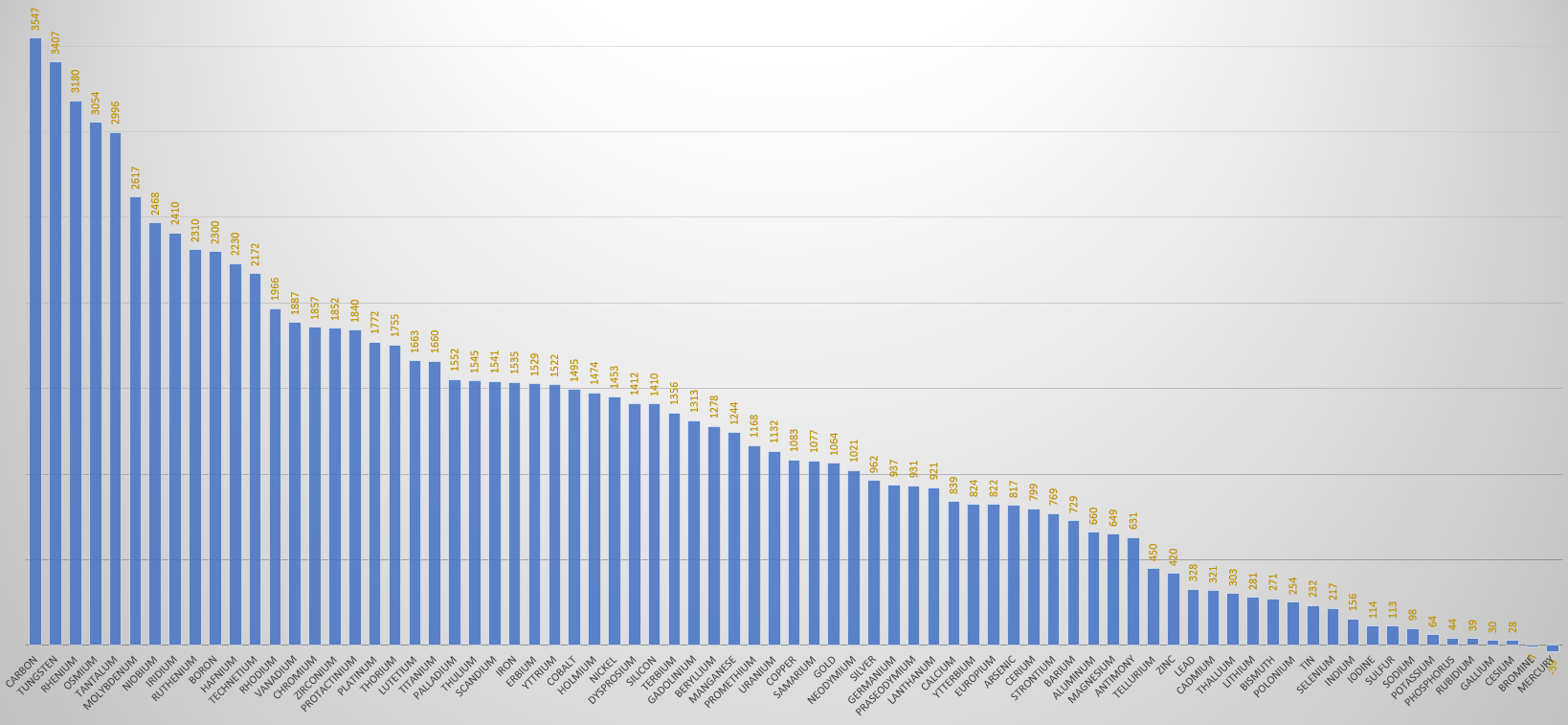
The following table of electron shells and subshells was generated by dumping Mathematica's ElementData["ElectronConfigurationString"] output into a text file, which was then passed through a script of C# code to convert it into a HTML <table>. It's a simple but effective visualisation of the order in which electrons progressively fill the subshells as the atomic number increases. The Aufbau Principle specifies that electrons prefer to fill lower energy levels first, so you would expect them to fill the shells in a predictable clockwork manner, but nature isn't quite that simple and irregularities appear in increasing numbers as the atomic number increases. Note the first hiccup at Chromium (Z=24) where a 4s electron has been borrowed by the 3d shell and its count jumps by 2 before returning to the expected pattern. The Aufbau article lists the element subshells where the pattern is broken (highlighted in blue below), and it discusses the subtleties of the subject in more detail. Element names in red are radioactive.
| 1s | 2s | 2p | 3s | 3p | 3d | 4s | 4p | 4d | 4f | 5s | 5p | 5d | 5f | 6s | 6p | 6d | 7s | 7p | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | H | Hydrogen | S | ||||||||||||||||||
| 2 | He | Helium | SS | ||||||||||||||||||
| 3 | Li | Lithium | SS | S | |||||||||||||||||
| 4 | Be | Beryllium | SS | SS | |||||||||||||||||
| 5 | B | Boron | SS | SS | P | ||||||||||||||||
| 6 | C | Carbon | SS | SS | PP | ||||||||||||||||
| 7 | N | Nitrogen | SS | SS | PPP | ||||||||||||||||
| 8 | O | Oxygen | SS | SS | PPPP | ||||||||||||||||
| 9 | F | Fluorine | SS | SS | PPPPP | ||||||||||||||||
| 10 | Ne | Neon | SS | SS | PPPPPP | ||||||||||||||||
| 11 | Na | Sodium | SS | SS | PPPPPP | S | |||||||||||||||
| 12 | Mg | Magnesium | SS | SS | PPPPPP | SS | |||||||||||||||
| 13 | Al | Aluminum | SS | SS | PPPPPP | SS | P | ||||||||||||||
| 14 | Si | Silicon | SS | SS | PPPPPP | SS | PP | ||||||||||||||
| 15 | P | Phosphorus | SS | SS | PPPPPP | SS | PPP | ||||||||||||||
| 16 | S | Sulfur | SS | SS | PPPPPP | SS | PPPP | ||||||||||||||
| 17 | Cl | Chlorine | SS | SS | PPPPPP | SS | PPPPP | ||||||||||||||
| 18 | Ar | Argon | SS | SS | PPPPPP | SS | PPPPPP | ||||||||||||||
| 19 | K | Potassium | SS | SS | PPPPPP | SS | PPPPPP | S | |||||||||||||
| 20 | Ca | Calcium | SS | SS | PPPPPP | SS | PPPPPP | SS | |||||||||||||
| 21 | Sc | Scandium | SS | SS | PPPPPP | SS | PPPPPP | D | SS | ||||||||||||
| 22 | Ti | Titanium | SS | SS | PPPPPP | SS | PPPPPP | DD | SS | ||||||||||||
| 23 | V | Vanadium | SS | SS | PPPPPP | SS | PPPPPP | DDD | SS | ||||||||||||
| 24 | Cr | Chromium | SS | SS | PPPPPP | SS | PPPPPP | DDDDD | S | ||||||||||||
| 25 | Mn | Manganese | SS | SS | PPPPPP | SS | PPPPPP | DDDDD | SS | ||||||||||||
| 26 | Fe | Iron | SS | SS | PPPPPP | SS | PPPPPP | DDDDDD | SS | ||||||||||||
| 27 | Co | Cobalt | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDD | SS | ||||||||||||
| 28 | Ni | Nickel | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDD | SS | ||||||||||||
| 29 | Cu | Copper | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | S | ||||||||||||
| 30 | Zn | Zinc | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | ||||||||||||
| 31 | Ga | Gallium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | P | |||||||||||
| 32 | Ge | Germanium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PP | |||||||||||
| 33 | As | Arsenic | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPP | |||||||||||
| 34 | Se | Selenium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPP | |||||||||||
| 35 | Br | Bromine | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPP | |||||||||||
| 36 | Kr | Krypton | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | |||||||||||
| 37 | Rb | Rubidium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | S | ||||||||||
| 38 | Sr | Strontium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | SS | ||||||||||
| 39 | Y | Yttrium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | D | SS | |||||||||
| 40 | Zr | Zirconium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DD | SS | |||||||||
| 41 | Nb | Niobium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDD | S | |||||||||
| 42 | Mo | Molybdenum | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDD | S | |||||||||
| 43 | Tc | Technetium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDD | SS | |||||||||
| 44 | Ru | Ruthenium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDD | S | |||||||||
| 45 | Rh | Rhodium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDD | S | |||||||||
| 46 | Pd | Palladium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | ||||||||||
| 47 | Ag | Silver | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | S | |||||||||
| 48 | Cd | Cadmium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | |||||||||
| 49 | In | Indium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | P | ||||||||
| 50 | Sn | Tin | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | PP | ||||||||
| 51 | Sb | Antimony | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | PPP | ||||||||
| 52 | Te | Tellurium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | PPPP | ||||||||
| 53 | I | Iodine | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | PPPPP | ||||||||
| 54 | Xe | Xenon | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | ||||||||
| 55 | Cs | Cesium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | S | |||||||
| 56 | Ba | Barium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | SS | |||||||
| 57 | La | Lanthanum | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | D | SS | ||||||
| 58 | Ce | Cerium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | F | SS | PPPPPP | D | SS | |||||
| 59 | Pr | Praseodymium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFF | SS | PPPPPP | SS | ||||||
| 60 | Nd | Neodymium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFF | SS | PPPPPP | SS | ||||||
| 61 | Pm | Promethium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFF | SS | PPPPPP | SS | ||||||
| 62 | Sm | Samarium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFF | SS | PPPPPP | SS | ||||||
| 63 | Eu | Europium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFF | SS | PPPPPP | SS | ||||||
| 64 | Gd | Gadolinium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFF | SS | PPPPPP | D | SS | |||||
| 65 | Tb | Terbium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFF | SS | PPPPPP | SS | ||||||
| 66 | Dy | Dysprosium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFF | SS | PPPPPP | SS | ||||||
| 67 | Ho | Holmium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFF | SS | PPPPPP | SS | ||||||
| 68 | Er | Erbium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFF | SS | PPPPPP | SS | ||||||
| 69 | Tm | Thulium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFF | SS | PPPPPP | SS | ||||||
| 70 | Yb | Ytterbium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | SS | ||||||
| 71 | Lu | Lutetium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | D | SS | |||||
| 72 | Hf | Hafnium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DD | SS | |||||
| 73 | Ta | Tantalum | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDD | SS | |||||
| 74 | W | Tungsten | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDD | SS | |||||
| 75 | Re | Rhenium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDD | SS | |||||
| 76 | Os | Osmium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDD | SS | |||||
| 77 | Ir | Iridium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDD | SS | |||||
| 78 | Pt | Platinum | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDD | S | |||||
| 79 | Au | Gold | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | S | |||||
| 80 | Hg | Mercury | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | |||||
| 81 | Tl | Thallium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | P | ||||
| 82 | Pb | Lead | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PP | ||||
| 83 | Bi | Bismuth | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PPP | ||||
| 84 | Po | Polonium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PPPP | ||||
| 85 | At | Astatine | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PPPPP | ||||
| 86 | Rn | Radon | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | ||||
| 87 | Fr | Francium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | S | |||
| 88 | Ra | Radium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | SS | |||
| 89 | Ac | Actinium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | D | SS | ||
| 90 | Th | Thorium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DD | SS | ||
| 91 | Pa | Protactinium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FF | SS | PPPPPP | D | SS | |
| 92 | U | Uranium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFF | SS | PPPPPP | D | SS | |
| 93 | Np | Neptunium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFF | SS | PPPPPP | D | SS | |
| 94 | Pu | Plutonium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFF | SS | PPPPPP | SS | ||
| 95 | Am | Americium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFF | SS | PPPPPP | SS | ||
| 96 | Cm | Curium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFF | SS | PPPPPP | D | SS | |
| 97 | Bk | Berkelium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFF | SS | PPPPPP | SS | ||
| 98 | Cf | Californium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFF | SS | PPPPPP | SS | ||
| 99 | Es | Einsteinium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFF | SS | PPPPPP | SS | ||
| 100 | Fm | Fermium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFF | SS | PPPPPP | SS | ||
| 101 | Md | Mendelevium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFF | SS | PPPPPP | SS | ||
| 102 | No | Nobelium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | SS | ||
| 103 | Lr | Lawrencium | SS | SS | PPPPPP | SS | PPPPPP | DDDDDDDDDD | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | DDDDDDDDDD | FFFFFFFFFFFFFF | SS | PPPPPP | SS | P |
Elements (82)
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Contents generated on machine OWL by Hoarder 8.0.31.0 on Monday, 01 December 2025 16:59 GMT+11:00

